- 10995 Quivira Road, Overland Park, Kansas 66210, United States
- Mon - Fri 8am - 5pm
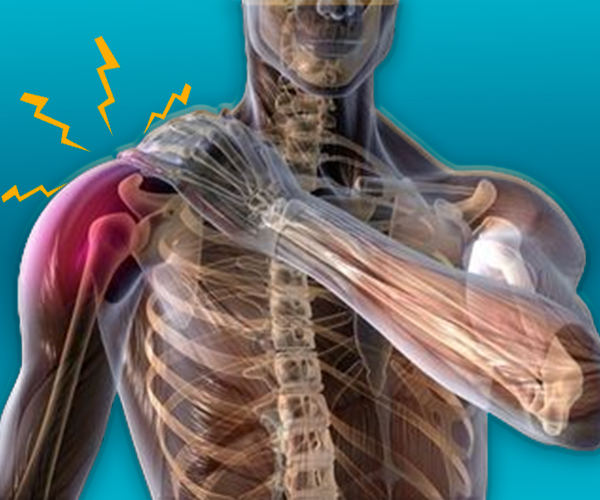
Research into chronic moderate to severe post-surgical neuropathic pain is essential to improve the quality of life for individuals who suffer from this condition. Here’s a general outline of a study design for such a research project
Length of study: 45 weeks (up to 5 patch applications)
Inclusion Criteria:
Exclusion Criteria:
We love our patients, so feel free to call during normal business hours and ask about current trials.
Overland Park, Kansas - (Kansas City area)
10995 Quivira Road, Overland Park, Kansas 66210, United States